Considering the amount of sometimes conflicting articles in the popular press recently, I thought I’d weigh in (pun intended) on the subject of the skinny jabs… what exactly are they, how do they work, and how do they fit in with the general health care practices?
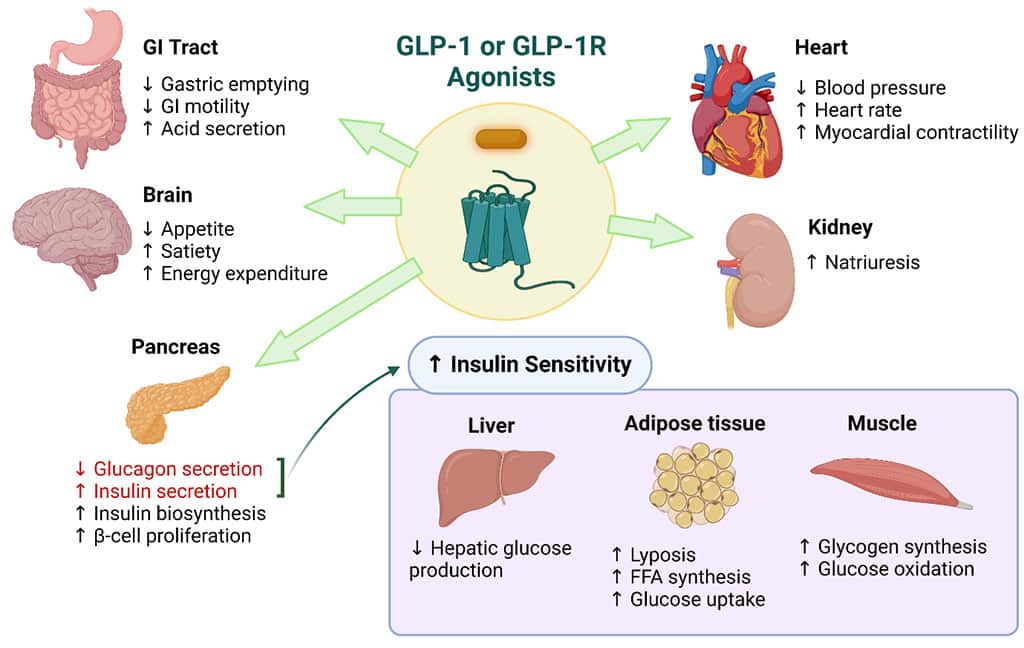
All of these weight loss medications fall under the pharmaceutical umbrella of Glucagon-like Peptide 1 (GLP-1) receptor agonists and were primarily developed as an anti-diabetic drug. These peptides can stimulate the pancreas to produce more insulin after meals, thus controlling sugar levels. They also slow down the transit of food from the stomach to the small intestine, which helps suppress appetite, leaving one feeling full faster and longer, thus reducing the total amount of food consumed.
Other health benefits include cardioprotective properties, and newer studies propose a potential positive influence on hormonal balance, lipid metabolism, and inflammatory pathways, offering potential therapeutic benefits in reproductive health and menopause-related and poly cystic ovary syndrome (PCOS) related metabolic changes. Furthermore, they have shown promise in reducing cancer risk, particularly in hormone-related malignancies such as breast and ovarian cancers.
The first GLP-1 agonist was actually “discovered” in 1984, but it wasn’t until 2005 that the FDA approved BYETTA -exenatide for the treatment of type 2 Diabetes Mellitus. Next came VICTOZA-liraglutide in 2010, followed by TRULICITY-dulaglutide in 2012, OZEMPIC-semaglutide in 2017 and finally MOUNJARO-tirzepatide in 2022. All of these were strictly approved as anti-diabetic agents.
However, as data from longer term studies started to flow in, the significant weight loss and the relative lack of hypoglycaemic episodes in patients taking these medications opened the door for liraglutide to be rebranded as SAXENDA and approved as a weight loss drug in 2014. The same happened for semaglutide, appearing as WEGOVY in 2021, and the subsequent astronomic rise in their use has been unprecedented. Currently, it is estimated that almost 12% of the USA population alone has taken these meds at some stage and almost 6% of the population is being prescribed them on a long-term basis. And that does not even take into account the black market “prescribers” and suppliers!
Both liraglutide and semaglutide were developed by Novo Nordisk, a Danish pharmaceutical company (currently worth €250 billion, down from over €500 billion), who have singlehandedly managed to increase the total GDP of the country by almost 3% through the sales of these two pharmaceuticals alone!
When properly supervised, the average expected weight loss while takings GLP-1 agonists in most patients is around 7% of their BMI, but this can go up to 14% and >20% with the newer products. But we must bear in mind that these studies were conducted on patients who were deemed clinically obese, with an average BMI of > 35.
Tabloids and social media pages have reported phenomenal weight losses achieved in timescales of less than six months, but such results often come at a significant cost to the patient, not just from a monetary perspective but also health wise. Such dramatic results are only achievable through high doses being administered very early in the weight loss journey, which goes completely against all guidelines from the medical fraternity. The most common side effects such as chronic nausea, vomiting and diarrhoea, constipation and abdominal pain affect 50-70% of all users, and can lead to acute pancreatic and liver disease if not properly monitored and their use suspended timeously. Excessive vomiting has been recorded in GLP-1 medicated patients undergoing anaesthesia, which can lead to an increased risk of complications such as aspiration pneumonia. Although these gastro-intestinal effects appear to be dose-related and resolve on stopping the medication, the relative paucity of long-term safety data is directly proportionate to the novelty of GLP-1 agonists.
New evidence emerging from longer-term studies published in the 2024 The Lancet Diabetes & Endocrinology, showed that muscle loss with GLP-1 agonists accounted for up to 39% of total weight lost, as compared to 10-30% with a normal calorie-restricted diet. This finding could potentially play a role in the weight gain reported on stopping the meds, as low muscle mass = low basal metabolic rate = tendency to put on weight.
The loss of muscle mass together with the proven lipolytic (fat burning) properties of these meds has resulted in the rise of what Plastic Surgeons are now calling the “Ozempic face” phenomenon. The resultant facial changes characterized by gauntness, sunken cheeks, new wrinkles and loose skin on the face and neck, have created an increased demand for facial rejuvenation surgeries.
Loss of muscle mass (especially in women), is also linked to higher risk of falls and hip fractures, causing more clinicians to now actively emphasize fat loss vs weight loss in patients taking GLP-1s.
So overall, just like any other medication, GLP-1 agonists have their pro’s and con’s, and just like any other prescription medication … and the emphasis here is on the word PRESCRIPTION MEDICATION … should be prescribed and monitored by a responsible medical professional who has done their due diligence on these medications and is comfortable supporting their patients on their weight loss journey.